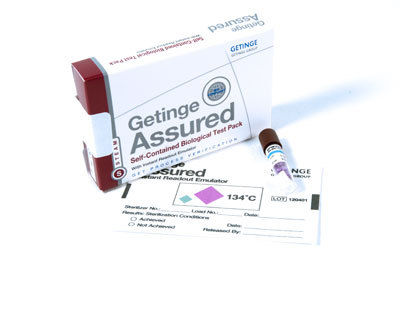
Self-Contained Biological Test Pack
5000 INR/Piece
Product Details:
- Capacity 1 test pack per cycle
- Accuracy High sensitivity for sterilization efficacy
- Core Components Self-contained biological indicator, chemical indicator card, packaging
- Model No SCBI PACK-121/135
- Measurement Range Log10 reduction of biological indicator spores
- Temperature Range 121C to 135C
- Automation Grade Manual
- Click to view more
X
Self-Contained Biological Test Pack Price And Quantity
- 5000 INR/Piece
- 1 Piece
Self-Contained Biological Test Pack Product Specifications
- Steam sterilization load monitoring
- Log10 reduction of biological indicator spores
- Approx. 90 x 60 x 25 mm
- 121C to 135C
- Manual
- Single-use per sterilization cycle
- Rapid readout, easy interpretation, consistent results
- Plastic and Chemical Indicator
- SCBI PACK-121/135
- High sensitivity for sterilization efficacy
- Self-contained biological indicator, chemical indicator card, packaging
- Approx. 30-50 g
- 1 test pack per cycle
- Color change indicator
- Self-Contained Biological Test Pack
Self-Contained Biological Test Pack Trade Information
- 5-10 Piece Per Month
- 6-8 Week
Product Description
Seize this Bargainour Self-Contained Biological Test Pack, engineered for Supreme reliability, is Ending Soon! With peerless accuracy and monumental performance, this test pack delivers rapid color indication within 24 hours, ensuring effective steam sterilization monitoring. Precision-designed with Geobacillus stearothermophilus spores, it supports a Sterility Assurance Level of 10. Each individually reserved pack is tamper-evident, compliant with ISO 11138 and EN 867-5, and easy to interpret visually. Reserve now to benefit from consistent results, supreme sensitivity, and hassle-free application in hospitals and operating theatres.
Versatile Applications in Healthcare Facilities
The Self-Contained Biological Test Pack is indispensable in hospitals Central Sterile Supply Departments (CSSD), operating theatres, and laboratories. Commonly used by healthcare professionals and equipment validation teams, it verifies the effectiveness of steam sterilization cycles. Its robust design ensures accurate and reliable monitoring of sterilizer loads, supporting regulatory compliance and patient safety. Whether you are validating autoclaves or routine sterilization processes, its monumental sensitivity and rapid indication make it the supreme choice.
FOB Port, Packaging & Supply Capacity
With a premium Outlay, our Self-Contained Biological Test Pack is dispatched from renowned Indian FOB ports. Shipped Goods are securely packaged, each pack individually tamper-evident, safeguarding integrity during transit. Packaging details are meticulously managed for safe delivery. The supply ability supports robust demand, ensuring timely delivery to manufacturers, suppliers, exporters, and importers. Take advantage of peerless logistics for prompt and reliable shipment, keeping your sterilization routines uninterrupted.
Versatile Applications in Healthcare Facilities
The Self-Contained Biological Test Pack is indispensable in hospitals Central Sterile Supply Departments (CSSD), operating theatres, and laboratories. Commonly used by healthcare professionals and equipment validation teams, it verifies the effectiveness of steam sterilization cycles. Its robust design ensures accurate and reliable monitoring of sterilizer loads, supporting regulatory compliance and patient safety. Whether you are validating autoclaves or routine sterilization processes, its monumental sensitivity and rapid indication make it the supreme choice.
FOB Port, Packaging & Supply Capacity
With a premium Outlay, our Self-Contained Biological Test Pack is dispatched from renowned Indian FOB ports. Shipped Goods are securely packaged, each pack individually tamper-evident, safeguarding integrity during transit. Packaging details are meticulously managed for safe delivery. The supply ability supports robust demand, ensuring timely delivery to manufacturers, suppliers, exporters, and importers. Take advantage of peerless logistics for prompt and reliable shipment, keeping your sterilization routines uninterrupted.
FAQs of Self-Contained Biological Test Pack:
Q: How is the color change interpreted in the Self-Contained Biological Test Pack?
A: The test pack provides a visual color change within 24 hours, making interpretation straightforward. A distinct shift in color indicates the response, confirming the efficacy of the sterilization process.Q: What is the main benefit of using Geobacillus stearothermophilus spores in this pack?
A: Geobacillus stearothermophilus spores are highly resistant to steam sterilization, providing monumental accuracy and reliability. Their use ensures a Sterility Assurance Level of 10, aligning with global safety standards.Q: When is the Self-Contained Biological Test Pack recommended for use during sterilization cycles?
A: Use one test pack per steam sterilizer cycle for reliable monitoring. It is especially vital in hospital CSSD, operating theatres, and during periodic equipment validation to maintain supreme sterility standards.Q: Where should the test pack be stored for optimal preservation?
A: The pack should be stored in a dry environment between 2C and 25C. Proper storage helps maintain its sensitivity and shelf life, ensuring consistent test results throughout its usable period.Q: What is the process for using and assessing the pack?
A: Simply place the pack inside the sterilizer load, run the cycle, then check the indicator for a color change after 24 hours. The process is manual, rapid, and requires only visual assessment.Q: How does the rapid readout feature benefit users?
A: The rapid readout provides results within 24 hours, accelerating decision-making and workflow, enabling quick verification of sterilization performance for healthcare teams and facility managers.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese Get A Quote
Get A Quote 






